The Discovery of Leptin, the Hormone That Regulates Body Weight

Freidman, Jeffrey
A strain of mice called obese, or ob, was first discovered in 1950 by researchers at The Jackson Laboratory. A recessive mutation causes these mice to be massively obese: they weigh three times as much as normal mice, and their appetite is insatiable. Rockefeller molecular geneticist Jeffrey Friedman (1954 - ) wanted to find out how a defect in just one gene could have such profound effects on both the animal’s body weight and its feeding behavior. He began searching for the ob gene in the late 1980s using the then-new method of positional cloning. It took eight years, but in 1994 Friedman achieved success: he cloned the ob gene in mice and its homolog in humans. In 1995 he purified the gene product, a hormone he called leptin.
Friedman’s discovery of leptin showed that there is a robust physiologic system that regulates food intake and metabolism, that fat is an endocrine organ, and that obesity is a problem of biology. Leptin acts to maintain homeostatic control of fat mass as follows. Leptin is secreted by fat cells into the bloodstream and acts on the brain to regulate food intake and energy expenditure. When fat mass falls, plasma leptin levels fall, stimulating appetite and suppressing energy expenditure until fat mass is restored. When fat mass increases, leptin levels increase, suppressing appetite until weight is lost. This system maintains homeostatic control of adipose tissue mass.
Leptin has been developed as a therapy for some obese people, and for treating the life-threatening metabolic disorder lipodystrophy, other forms of diabetes, and hypothalamic amennorhea. Friedman’s landmark papers also unleashed a flood of research in laboratories around the world: since leptin’s discovery, nearly 30,000 articles have been published on the hormone.
Leptin, which is made by the body’s fat cells, is the linchpin in a complex endocrine system that not only maintains the body’s weight in a narrow range, but also exerts powerful effects on other aspects of physiology related to an organism’s nutritional state: for example, the entire neuro-endocrine axis including reproduction, glucose metabolism and insulin sensitivity, and immune status. Friedman found that the mice with the mutated gene for leptin completely lacked leptin, so they ate until they became obese because their brains perceived themselves as starving. When given leptin supplements, the mice ate less, lost weight, became more active, and also responded better to insulin. Similarly, humans with leptin mutations who fail to produce leptin also are massively obese and show dramatic weight loss with leptin therapy.
However, not all obese people have these mutations. In studies with human subjects, only a subset of obese people lost weight with leptin therapy. It turned out that most obese people produce plenty of leptin, but their bodies fail to respond to it—rather than leptin deficiency, obesity is most often due to leptin resistance. More recently, a possible means for overcoming leptin resistance has been suggested by the results of a study testing the effect of a combination of leptin with another hormone, amylin. This combination has shown promise in clinical studies of obese patients: obese patients receiving both hormones lost an average of 13 percent of their weight.
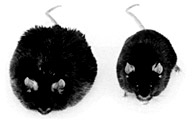
Ob/ob mouse. Copyright 1995 Amgen Inc.
Friedman has continued both laboratory studies and clinical research on the mechanisms by which leptin controls feeding behavior and body weight. One focus of his lab is the molecular pathways by which leptin regulates the relevant neural pathways in the hypothalamus. Detailed studies of these circuits has revealed a surprising plasticity in response to leptin treatment, suggesting that the wiring of neural circuits that control feeding may be different in the lean and the obese. Clinical studies are investigating the effects of leptin on obese patients as they lose weight on a low-calorie diet. Friedman also is searching for more genes that contribute to obesity in a collaborative project with other researchers and with the Department of Health on the Pacific Island of Kosrae in Micronesia.
Jeffrey M. Friedman received the BS from Rensselaer Polytechnic Institute (1973) and the MD from Albany Medical College of Union University (1977). After completing two residencies at Albany Medical Center Hospital, he came to Rockefeller as a postgraduate fellow and associate physician in 1980. In 1986 he received his PhD from Rockefeller, working in the laboratory of James E. Darnell Jr., and was appointed assistant professor. Friedman was named head of laboratory in 1991, professor in 1995, and Marilyn M. Simpson Professor in 1999. He has been an investigator at the Howard Hughes Medical Institute since 1986. Among many awards and honors, Friedman’s achievements have been recognized by the Bristol Myers Squibb Award for Distinguished Achievement in Metabolic Research (2001), the Passano Foundation Award (2004), the Gairdner Foundation International Award (2004), the Jessie Stevenson Kovalenko Medal (2007), the Danone International Prize for Nutrition (2007), the Shaw Prize (2009), and the Keio Medical Science Prize (2009). He is an elected member of the U.S. National Academy of Sciences (2001) and its Institute of Medicine (2005).
Selected Publications
Zhang Y, Proenca R, Maffel M, Barone M, Leopold L, and Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature, 1994, 372: 425-432
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, and Friedman JM. Weight-reducing effects of the plasma-protein encoded by the obese gene. Science, 1995, 269: 543-546
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, and Friedman JM. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight reduced subjects. Nature Medicine, 1995, 1: 1155-1161
Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, and Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature, 1996, 379: 632-635
Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, and Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science, 2004, 304: 110-115
Further Reading
Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr, 2009, 89(suppl): 973S-979S
Friedman JM. A war on obesity, not the obese. Science, 2003, 299: 856–858.
Friedman JM. Modern science versus the stigma of obesity. Nat Med, 2004, 10: 563-569
Links
Jeffrey M. Friedman, Laboratory of Molecular Genetics
http://www.rockefeller.edu/labheads/friedman/friedman-lab.php
Jeffrey M. Friedman, HHMI Investigator
http://www.hhmi.org/research/investigators/friedman.html
Interview: Jeffrey Friedman Discusses Obesity Research
http://www.hhmi.org/biointeractive/media/friedman_bio-lg.mov
HHMI Bulletin: Leptin’s Legacy
http://www.hhmi.org/bulletin/mar2003/leptin/