Unraveling the Link Between Immunologic Control of Cancer and Severe Neurologic Disease—and Harnessing This Knowledge for Cancer Therapy

Darnell, Robert
People with the rare degenerative brain diseases called paraneoplastic neurologic disorders (PND) suffer a sudden onset of severe symptoms, ranging from vertigo and loss of muscle coordination to blindness. When they visit the doctor, they get a second, unexpected diagnosis: cancer. In PND, the immune system mounts an assault that often holds an underlying cancer at bay, so that people are not aware of it. But this immune response also attacks the brain, because the tumor cells make a protein, or antigen, identical to one normally expressed only by certain brain cells. When Robert B. Darnell (1957-) first observed patients with PND in the clinic in 1987, an immunological link between cancer and PND — an antibody in patients' blood and spinal fluid — had only recently been discovered. Through studies with patients at the Rockefeller Hospital, Darnell has since unraveled the key steps in this immune response, providing groundbreaking evidence that humans have a natural immune response to tumors. Furthermore, he has turned the process back on itself to develop experimental vaccines that may be effective against a variety of cancers, including prostate cancer.
Darnell initially used the tools of molecular biology to analyze antibodies in the serum of people with PND. His laboratory identified the first gene coding for a corresponding antigen present both in tumor cells and normal brain cells, paving the way for the discovery of more than a dozen such genes. Knowing the genes allowed for accurate diagnosis of different types of PND (an antibody called Yo, for example, is linked to cerebellar degeneration and breast and ovarian cancers). Then Darnell's research group turned to the question, what generates the cellular response of the immune system — the killer T cells specific to the antigens found on patients' tumor cells? They found that when tumor cells undergo a specific form of death (apoptosis), they pass these antigens to immune system cells called dendritic cells, which in turn activate the T cells. By mimicking this process, Darnell is developing vaccines that aim to prime a cancer patient's immune system to attack tumors. For example, Darnell has taken prostate tumor cells, made them undergo apoptosis, and then fed them to dendritic cells. These dendritic cells are then the basis for a vaccine, in early-phase clinical studies, that signals a patient's T cells to find the cancer.
Another approach to therapy involves transforming normal T cells, grown in laboratory culture, into tumor killers that target breast and ovarian cancers when administered to patients. T cells recognize these tumors not only by a particular antigen, called cdr2, but also by a section of a "docking" protein termed MHC I on the surface of the tumor cells. Darnell's team isolated the genes for the T-cell receptor that recognizes fragments of the cdr2 antigen on tumor MHC I molecules, then injected them into normal human T cells. As a result, the transfected T cells (CD8+ T cells) express the cdr2-specific T cell receptor, allowing them in turn to recognize and kill tumor cells in tissue culture that express cdr2, suggesting a strategy for treating these tumors.
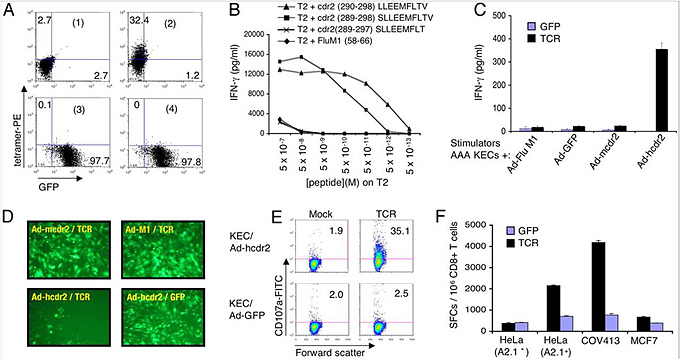
Characterization of a cdr2-specific T-cell receptor. From PNAS, 2007, 104: 19073-19078
Darnell's lab is also interested in what the PND antigens are — what is their normal role in neurons, and why do tumors induce their expression? These questions have led them to explore the function of neuron-specific RNA-binding proteins in neuronal biology and in disease, including the PND antigens Hu and Nova, and the related protein FMRP — the protein mutated in fragile X mental retardation. Recently, the lab has developed a general technique called high throughput sequencing-cross linking immunoprecipitation, or HITS-CLIP, to create genome-wide maps of where RNA-binding proteins bind to RNA in living tissue. HITS-CLIP, together with analysis of knockout mice and bioinformatics, has been able to predict and validate rules governing alternative splicing and polyadenylation in vivo. For example, by studying Nova in detail, the lab has determined that Nova coordinately regulates transcripts encoding synaptic proteins, and has identified the way in which it does so. The lab is now extending their approach to develop functional insight into how RNA regulation — including that mediated by FMRP and Argonaut/microRNA complexes — function in isolation and in combination within living cells. This work is being applied to the study of human disease, and to physiology, with a new focus emanating from a series of findings relating Nova RNA regulation to the balance of neuronal inhibition and excitation, and motor neuron function in the spinal cord. The work could lead to the identification of new relationships between the regulation of RNA complexity in the nucleus and the complexity of the cell itself.
Robert B. Darnell received the BA from Columbia University (1979) and the MD and PhD from Washington University School of Medicine in St. Louis (1985). He trained in internal medicine at Mount Sinai School of Medicine and in neurology at Cornell Medical College. In 1990, he began clinical work at Memorial Sloan-Kettering Cancer Center, where he continues to teach as an attending neurologist. He is also on the associate faculty of Weill Medical College of Cornell University in neurology and neuroscience. Darnell joined Rockefeller University in 1992, and was appointed professor and senior physician in 2000. In 2002 Darnell became an investigator at the Howard Hughes Medical Institute and was named the Heilbrunn Professor at Rockefeller. His achievements have been recognized with the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research (2000), the Derek Denny-Brown Young Neurological Scholar Award (1998), and the Irma T. Hirschl Career Scientist Award (1996).
Selected Publications
Darnell RB and DeAngelis LM. Regression of small-cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet, 1993, 341: 21-22
Newman LS, McKeever MO, Okano HJ and Darnell RB. Î2-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell, 1995, 82: 773-783
Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: At the intersection of cancer, immunity and the brain. Proc Natl Acad Sci USA, 1996, 93: 4529-4536
http://www.pnas.org/content/93/10/4529.full.pdf+html
Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, and Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med, 1998, 4: 1321-1324
Darnell JC, Albert ML, and Darnell RB. Cdr2, a target antigen of naturally occurring human tumor immunity, is widely expressed in gynecological tumors. Cancer Res, 2000, 60: 2136-2139
Santomasso BD, Roberts WK, Thomas A, Williams T, Blachère NE, Dudley ME, Houghton AN, Posner JB, and Darnell RB. A T-cell receptor associated with naturally occurring human tumor immunity. Proc Natl Acad Sci USA, 2007, 104: 19073-19078
http://www.pnas.org/content/104/48/19073.full.pdf+html
Darnell JC, Jensen KB, Brown V, Jin P, Warren ST, and Darnell RB. Fragile X mental retardation protein targets G-quartet mRNAs important for neuronal function. Cell, 2001, 107:489-499
Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, and Darnell RB. CLIP identifies Nova-regulated RNA networks in the brain. Science, 2003, 302:1212-1215
Darnell JC, Fraser CE, Mostovetsky O, Stefani G, and Darnell RB. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev, 2005, 19: 903-918
Ule J, Ule A, Spencer J, Williams A, Hu J-S, Cline M, Wang H, Clark T, Fraser C, Ruggiu M, Zeeberg BR, Kane D, Weinstein JN, Blume J, and Darnell RB. Nova regulates neuronal splicing to shape synaptic activity. Nature Genet, 2005, 37:844-852; Epub 2005 Jul 24
Ule J, Stefani G, Mele A, Ruggiu M, Wang X, Taneri B, Gaasterland T, Blencowe BJ, and Darnell RB. An RNA map predicting Nova-dependent splicing regulation. Nature, 2006, 444: 580-586
Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, Clark TA, Schweitzer AC, Blume JE, Wang X, Darnell JC, and Darnell RB. HITS-CLIP yields genome-wide insight into brain alternative RNA processing. Nature, 2008, 456: 464-469
Chi SW, Zang JB, Mele A, and Darnell RB. Ago HITS-CLIP decodes miRNA-mRNA interaction maps. Nature, 2009, 460: 479-486
Further Reading
Licatalosi DD and Darnell RB. RNA processing and its regulation: global insights into biological networks. Nature Rev Genetics, 2010, 11: 75-87
Darnell RB. Developing global insight into RNA regulation. Cold Spring Harb Symp Quant Biol, 2006, 71: 321-327
Ule J and Darnell RB. RNA binding proteins and the regulation of neuronal synaptic plasticity. Curr Opin Neurobiol, 2006, 16: 102-110
Licatalosi DD and Darnell RB. Splicing regulation in neurologic disease. Neuron, 2006, 314: 126-129
Albert ML and Darnell RB. Paraneoplastic neurological degenerations: Keys to tumour immunity. Nature Rev Cancer, 2004, 4: 36-44
Roberts WK and Darnell RB. Neuroimmunology of the paraneoplastic neurological degenerations. Curr Opinion Immunol, 2004, 16: 616-622
Mead JC. When cancer is just the beginning. The Scientist, 2008, 22: 38
http://www.the-scientist.com/article/display/55048/
Links
The Laboratory of Molecular Neuro-Oncology at The Rockefeller University
http://www.rockefeller.edu/labheads/darnellr/
Howard Hughes Medical Institute research summary
http://www.hhmi.org/research/investigators/darnell.html