Endocrine-gene Interactions in the Hereditary Liver Disease, Acute Intermittent Porphyria

Kappas, Attallah
A broad program of laboratory and clinical studies of hepatic heme metabolism in humans was initiated by Attallah Kappas (1926- ) when he established the Laboratory of Pharmacology at The Rockefeller University Hospital in 1967. Laboratory research involved studies on key enzymes in heme synthesis, utilization and catabolism in the liver with a principal clinical focus on the hereditary and acquired disorders of the heme pathway, known as the porphyrias — particularly Acute Intermittent Porphyria (AIP), a genetic liver disease whose disabling neurologic symptoms may become lethal. The Rockefeller Hospital with its unique clinical and research resources was critical for the conduct of these studies and for the complex care of porphyria patients.
Kappas was interested in endocrine-hepatic interactions, and noted that patients with AIP commonly tended to be female, and that hormonal events, e.g. puberty, the menstrual cycle, and sex steroid use, triggered acute exacerbations of the disease. These clinical observations led him to the laboratory, and to the use of cultured avian embryonic hepatic cells to examine the possible actions of natural steroids on heme pathway enzymes in liver. Working initially with Sam Granick, a plant physiologist at Rockefeller (heme and chlorophyll share several enzymic steps in their early syntheses), Kappas found that steroids of a particular structure strongly induced, in the cultured liver cells, overactivity of ALA-synthase (ALAS), the rate-controlling enzyme in heme synthesis. Overactivity of hepatic ALAS also characterizes AIP patients who are experiencing an acute exacerbation of their disorder. Since these patients carry additionally the primary gene defect of AIP (PBG deaminase deficiency), which impairs normal processing of the previously formed intermediate, porphobilinogen (PBG), through later steps in the heme pathway, overactivity of hepatic ALAS leads to accumulation of this unprocessed compound. Unfortunately, PBG is toxic to neural cells and is believed to cause the severe neurological symptoms and signs of acute AIP.
The fact that certain steroids natural to man could strongly induce ALAS in cultured liver cells suggested that interdicting the formation of such compounds in gene carriers of AIP might lead to amelioration of this disorder. This consideration led Kappas and his colleagues to the development of a new treatment approach for AIP patients experiencing cyclic attacks of the disease — suppression of endogenous steroid production by an LHRH agonist acting centrally in the pituitary to reduce hormone production. This treatment proved highly successful in moderating their disease and in preventing its repeated exacerbations. Beyond this clinical outcome, discovery of the role which natural steroids played in regulation of the key enzyme controlling heme synthesis was new to science and defined the liver as a major target organ of the endocrine system.
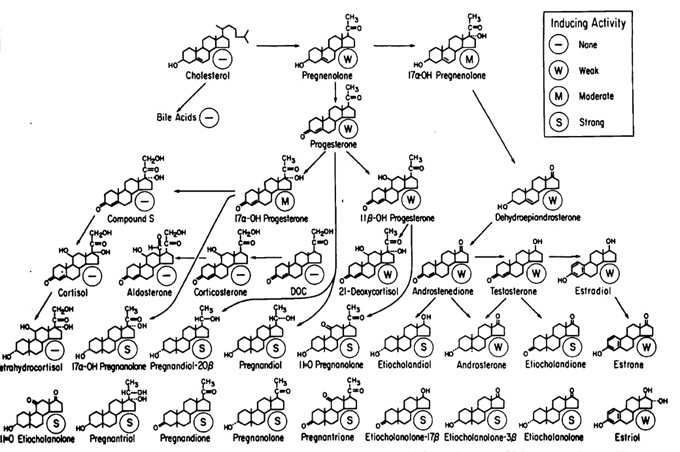
Summary of the principal metabolic pathways for the conversion of cholesterol to steroid hormones, intermediates, and metabolites.
From J Biol Chem, 1967, 242: 4587-4593
Attallah Kappas received an AB degree from Columbia (1947), following completion of his military service; and an MD degree from the University of Chicago (1950). He undertook post-graduate training at the Memorial Sloan-Kettering Cancer Center (MSKCC) and the Peter Bent Brigham Hospital, returning to Chicago in 1957. He was recruited to The Rockefeller University in 1967. In addition to heading the Laboratory of Pharmacology, he served successively during the period 1971-1991 as Program Director; Physician-in-Chief; and Vice President. He was a strong advocate of developing close collaborative relationships with Rockefeller's neighboring institutions and held for various periods joint appointments as the Vincent Astor Chair in Clinical Sciences at MSKCC; professorships in Medicine and Pharmacology at Cornell University Medical College; and Co-Directorship of the Rockefeller-Cornell Medical College MD-PhD program. He was a recipient of Commonwealth Fund and John Simon Guggenheim Memorial Foundation Fellowships; The Burroughs Wellcome Fund Special Award in Clinical Pharmacology; and the ASPET Award for Research in Therapeutics of the American Society of Pharmacology and Experimental Therapeutics. He served as an editor of the Journal of Experimental Medicine during 1971-1981. He was appointed the first Nicholson Exchange Professor from Rockefeller to the Karolinska Institute in 1985 and in 1989 became the first recipient of the newly established NIH Award for Excellence in Clinical Research. He is presently Sherman Fairchild Professor and Physician-in-Chief, Emeritus, at The Rockefeller University.
Selected Publications
Granick S and Kappas A: Steroid induction of porphyrin synthesis in liver cell culture. I. Structural basis and possible physiological role in the control of heme formation. J Biol Chem, 1967 242: 4587-4593
http://www.jbc.org/cgi/reprint/242/20/4587
Kappas A and Granick, S: Steroid induction of porphyrin synthesis in liver cell culture. II. The effects of heme, uridine diphosphate glucuronic acid, and inhibitors of nucleic acid and protein synthesis on the induction process. J Biol Chem, 1968 243: 346-351
http://www.jbc.org/cgi/reprint/243/2/346
Kappas A, Song CS, Levere RD, Sachson RA and Granick S. The induction of δ-aminolevulinic acid synthetase in vivo in chick embryo liver by natural steroids. Proc Natl Acad Sci USA, 1968, 61: 509-513
http://www.pnas.org/content/61/2/509.full.pdf+html
Kappas A, Bradlow HL, Gillette PN and Gallagher TF. Studies in porphyria I. A defect in the reductive transformation of natural steroid hormones in the hereditary liver disease, acute intermittent porphyria. J Exper Med 1972, 136: 1043-1053
http://jem.rupress.org/cgi/reprint/136/5/1043
Anderson KE, Spitz IM, Bardin CW, and Kappas A. A gonadotropin releasing hormone analogue prevents cyclical attacks of porphyria. Arch Int Med, 1990, 150: 1469-1474
Further Reading
Kappas, A, Sassa S, Galbraith RA, Drummond GS, and Nordman Y: The porphyrias. In The Metabolic and Molecular Bases of Inherited Disease, Seventh Edition, C.R. Scriver, A.L. Beaudet, W.S. Sly and D. Valle (eds.), McGraw-Hill Book Company, New York, 1995 Chapter 66: 2103-2159
Principal members of the Kappas laboratory group who participated in these research programs were: Nader G. Abraham, Alvito P. Alvares, Karl E. Anderson, David R. Bickers, Allan H. Conney, George S. Drummond, Richard A. Galbraith, Mahin D. Maines, Arlene B. Rifkind, Daniel W. Rosenberg, Mohinder K. Sardana, Shigeru Sassa and Takeo Yoshinaga.
Links
Attallah Kappas laboratory:
http://www.rockefeller.edu/labheads/kappas/kappas-lab.php
Learn More About the Laboratory of Pharmacology
At Rockefeller Attallah Kappas established the Laboratory of Pharmacology in the Hospital in 1967. His group focused on research in heme biology, and undertook a broadly based program of biochemical and clinical studies of the enzymes involved in heme synthesis and the porphyrias related to them; on heme utilization in the form of cytochrome P450 in liver and the regulation of P450-mediated drug and hormone metabolism by components of the human diet; and on the development and clinical application of synthetic heme analogues for downregulating the catabolism of heme to the bile pigment bilirubin. In each main direction of his biochemical research Dr. Kappas conducted a major program of clinical investigation. He also established at the Rockefeller Hospital a center for the diagnosis, care, and study of the diseases of porphyrin origin and, in collaborative relationships with other institutions, undertook similar efforts for studies of newborns and children with genetic or developmental forms of severe jaundice. Principal members of his laboratory group who joined in these research programs were: Nader G. Abraham, Alvito P. Alvares, Karl E. Anderson, David R. Bickers, Allan H. Conney, George S. Drummond, Richard A. Galbraith, Mahin D. Maines, Arlene B. Rifkind, Daniel W. Rosenberg, Mohinder K. Sardana, and Shigeru Sassa.