Discovering Genes that Regulate Circadian Rhythms

Young, Michael
All living things—plants, animals, insects, and even bacteria—keep time with their biochemistry and behavior in a 24-hour cycle. Fruit flies are particularly amenable to study with the methods of genetics, and in the early 1970s researchers discovered mutant fruit flies with altered rhythms of rest and activity. A decade later new recombinant DNA methods made it easier to isolate genes. Rockefeller geneticist Michael Young (1949 - ) cloned the period (per) gene of the fruit fly Drosophila melanogaster in 1984, the first gene known to regulate circadian rhythm. In addition to Young, two researchers then at Brandeis University, Jeffrey Hall and Michael Robash, also cloned the gene the same year.
Period turned out to be the first of several genes now known to make up the circadian clock. In the 1990s, Young identified per’s partner gene, timeless (tim) and found that the two genes work together in a feedback loop that oscillates on a 24-hour cycle. Young and colleagues went on to discover additional genes associated with the circadian clock—double-time, shaggy, vrille, and pdp1. Mutations in these genes affect behavior in Drosophila, for example by making the usual 24-hour cycle longer or shorter. Analogous clock genes have been identified in organisms ranging from the fungus Neurospora to fish, frogs, and mammals, including humans. Three of them have been associated with sleep disorders in humans. In addition, because many behavioral and physiological rhythms are controlled by circadian clocks, understanding their genetics can shed light on mood disorders, for example, and problems related to metabolism, learning, and memory.
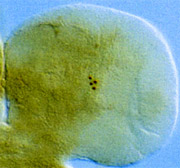
In Drosophila, four to five neurons (brown staining) produce circadian rhythms of period, timeless, Clock and vrille gene expression. From Laboratory of Genetics website.
Young and his colleagues are continuing their studies of the cellular and molecular machinery of circadian clocks in Drosophila, but their research has grown to include a focus on the rhythmic activities of genes and proteins in cells cultured from human subjects with delayed patterns of sleep. These studies have found evidence that naturally occurring molecular changes in the circadian clock can underlie varied patterns of human sleep.
Michael W. Young received his undergraduate degree (1971) and PhD (1975) from The University of Texas, Austin. Following postdoctoral work in biochemistry at Stanford University School of Medicine, he was appointed assistant professor at Rockefeller in 1978 as part of The Rockefeller University Fellows Program. He was named associate professor in 1984 and professor in 1988, and in 1991 he was appointed head of the Rockefeller Unit for the National Science Foundation’s Science and Technology Center for Biological Timing. He was named the university’s vice president for academic affairs and Richard and Jeanne Fisher Professor in 2004. Young was an investigator of the Howard Hughes Medical Institute from 1987 to 1996, and is a fellow of the American Academy of Microbiology and an elected member of the U.S. National Academy of Sciences (2007). In 2009 Young received the Neuroscience Prize of the Peter and Patricia Gruber Foundation.
Selected Publications
Bargiello TA and Young MW. Molecular genetics of a biological clock in Drosophila. Proc Natl Acad Sci USA, 1984, 81:2142-2146
http://www.pnas.org/content/81/7/2142.full.pdf+html
Baylies MK, Bargiello TA, Jackson FR, and Young MW. Changes in abundance or structure of the per gene product can alter periodicity of the Drosophila clock. Nature, 1987, 326: 390-392
Sehgal A, Price JL, Man B, and Young MW. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science, 1994, 263: 1603-1606
Vosshall, LB, Price JL, Sehgal A, Saez L, and Young MW. Block in nuclear localization of period protein by a second clock mutation, timeless. Science, 1994, 263: 1606-1609
Myers MP, Wager-Smith K, Wesley CS, Young MW, and Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science, 1995, 270: 805-808
Myers MP, WagerSmith K, RothenfluhHilfiker A, and Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science, 1996, 271: 1736-1740
Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, and Young, MW. Double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell, 1998, 94: 83-95
Blau J, and Young MW. Cycling vrille expression is required for a functional Drosophila clock. Cell, 1999, 99: 661-671
Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, Hardin PE, Young MW, Storti RV, and Blau J. vrille, Pdp1 and dClock form a second feedback loop in the Drosophila circadian clock. Cell, 2003, 112: 329-341
Meyer P, Saez L, and Young MW. PER-TIM interactions in living Drosophila cells: an interval timer for the circadian clock. Science, 2006, 311: 226-229
Further Reading
Young MW. The tick-tock of the biological clock. Scientific American, 2000, 282: 64-71
Young MW and Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet, 2001, 2: 702-715
Wijnen H and Young MW. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet, 2006, 40: 409-448
Links
Michael W. Young, Laboratory of Genetics
http://www.rockefeller.edu/labheads/young/young-lab.php
Clocks & Rhythms with Ulrich Schibler and Michael Young interviewed by Jan Witkowski
http://www.scivee.tv/node/10894