The Molecular Basis of Influenza and Paramyxoviruses Cell Penetration

Choppin, Purnell
To cause infection, a virus must enter a cell and direct its nucleic acid and protein synthesizing machinery to produce new viruses. Research led by Purnell Choppin (1929- ) in the 1960s and 1970s provided some of the first details of this process at the molecular level. His laboratory was among the first to design a specific antiviral agent based on knowledge of the structure of a viral protein. Choppin's research also advanced knowledge of viral virulence.
Samuel Dales, a researcher in the Porter and Palade Laboratory at Rockefeller, found in electron microscopic studies that several viruses are taken into cells by a process of endocytosis. In this process the cell engulfs the virus by wrapping its outer membrane around the virus particle and taking it into the cell inside a vesicle surrounded by the cell's invaginated membrane. Dales and Choppin showed that influenza virus entered cells via this mechanism. The question remained as to how the virus particle, or the internal proteins of the virus associated with the viral RNA, gained access to the cytoplasm of the cell to continue the infectious process. Studying the multiplication of influenza virus in cultured cells, Sondra Lazarowitz, a graduate student in Choppin's laboratory, had shown that the hemagglutinin protein (HA), which attaches the virus to the cell, was cleaved by a host cell proteolytic enzyme. Subsequent research in the Choppin laboratory and by others revealed that cleavage of the protein was not necessary for binding the virus to the cell; however, it was necessary for the penetration of the internal virus proteins and RNA into the cell's cytoplasm. This was accomplished by hemagglutinin-mediated fusion of the viral membrane with the cell membrane of the vesicle in which the virus resided after its endocytosis.
Work in the Choppin laboratory on a related group of viruses, the paramyxoviruses, aided the studies on influenza virus penetration. Andreas Scheid, Ming-chu Hsu, and others purified and characterized the paramyxovirus proteins. They then showed that, with these viruses, the virus binds to the outer cell membrane via one protein (HN), but another protein (F) is responsible for fusion of the viral membrane with the cell membrane, enabling the viral proteins associated with the infecting viral RNA to enter the cell. That membrane fusion step depends on the cleavage of the F protein by a host cell proteolytic enzyme. Thus, in both paramyxoviruses and influenza virus, the fusion of viral and cell membranes is due to a virus protein whose fusion activity depends on cleavage by a host protease. With paramyxoviruses this fusion occurs at the cell surface, whereas with influenza virus it occurs within the endocytic vesicle after endocytosis of the virus particle.
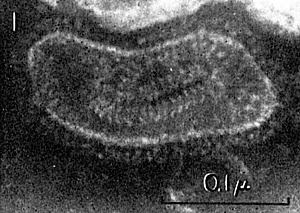
The Paramyxovirus SV5 with the internal nucleocapsid coiled inside. From Virology, 1964, 23(2): 195-202
The cleavage of the paramyxovirus F protein into two subunits, F1 and F2, creates a new end on F2, designated the fusion polypeptide. Analysis of the sequence of amino acids at this end of the polypeptide revealed a striking similarity among several viruses, and suggested that this end could penetrate the cell membrane. Based on these findings, Christopher Richardson, Andreas Scheid, and Choppin synthesized competitive inhibitors consisting of a series of amino acids similar to that on the end of the F2 polypeptide. These were highly active in preventing virus penetration and infection. Similar inhibitors synthesized on the same principle inhibited infection by influenza viruses. This provided an important early example of the design of a specific antiviral agent from knowledge of the structure of a viral protein.
Purnell Choppin received an MD degree from Louisiana State University School of Medicine (1953) and served as an intern and resident at Barnes Hospital, Washington University School of Medicine in St. Louis. After coming to the Rockefeller Institute in 1957, Choppin remained until 1985, and advanced from postdoctoral fellow to professor and senior physician to the Hospital. He also served as Vice President for Academic Programs and Dean of Graduate Studies. After leaving Rockefeller he joined the Howard Hughes Medical Institute where he served as President until retirement in 2000. He is a member of the U.S. National Academy of Sciences (1977) and the Institute of Medicine, the American Philosophical Society, and the American Academy of Arts and Sciences. His honors include the Howard Taylor Ricketts Award from the University of Chicago, the Selman A. Waksman Award for Excellence in Microbiology from the National Academy of Sciences, the Dean's Medal from Harvard Medical School, the University of California at San Francisco Medal, the Johns Hopkins University Heritage Award, and several honorary degrees from universities in the United States and abroad.
Selected Publications
Holmes KV and Choppin PW. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med, 1966, 124: 501-520
http://jem.rupress.org/cgi/reprint/124/3/501
Scheid A and Choppin PW. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity by proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology, 1974, 57: 475-490
Scheid A and Choppin PW. Protease activation mutants of Sendai virus: Activation of biological properties by specific proteases. Virology, 1976, 69: 265-277
Scheid A and Choppin PW. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology, 1977, 80: 54-66
Merz DC, Scheid A, and Choppin PW. The importance of antibodies to the fusion glycoprotein (F) of paramyxoviruses in the prevention of spread of infection. J Exp Med, 1980, 151: 275-288
http://jem.rupress.org/cgi/reprint/151/2/275
Richardson CD, Scheid A, and Choppin PW. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology, 1980, 105: 205-222
Hsu M-C, Scheid A, and Choppin PW. Activation of the Sendai virus fusion protein (F) involves a conformational change with exposure of a new hydrophobic region. J Biol Chem, 1981, 256: 3557-3563
http://www.jbc.org/content/256/7/3557.full.pdf+html
Hsu M-C, Scheid A, and Choppin PW. Fusion of Sendai virus with liposomes: dependence on the viral fusion protein (F) and the lipid composition of liposomes. Virology, 1983, 146: 361-369 1983.
Richardson CD and Choppin PW. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology, 1983, 131: 518-532
Hsu M-C, Scheid A, and Choppin PW. Protease activation mutants of Sendai virus: sequence analysis of the mRNA of the fusion protein (F) gene and direct identification of the cleavage-activation site. Virology, 1987, 156: 84-90
Further Reading
Scheid A and Choppin PW. Isolation of paramyxovirus glycoproteins and identification of their biological properties. In Negative Strand Viruses, Vol I. Barry RD and Mahy BWJ, eds. London: Academic Press, 1975, pp. 177-192
Scheid A and Choppin PW. Activation of cell fusion and infectivity by proteolytic cleavage of a Sendai virus glycoprotein. In Proteases and Biological Control, Reich E, Rifkin DB, and Shaw E, eds. Cold Spring Harbor Laboratory, 1975, pp. 645-659
Scheid A, Graves MC, Silver SM, and Choppin PW. Studies on the structure and functions of paramyxovirus glycoproteins. In Negative Strand Viruses and the Host Cell, Mahy BWJ and Barry RD, eds. London: Academic Press, 1978, pp. 181-193
Choppin PW, Richardson CD, and Scheid A. Oligopeptides as specific antiviral agents. In Targets for the Design of Antiviral Agents, Declercq E and Walker RT, eds. New York: Plenum, 1984, pp. 287-305