Management of Severe Newborn Jaundice: Changing the Clinical Paradigm from Treatment to Prevention

Kappas, Attallah
Jaundice is very common in newborns because their immature livers are not efficient at removing bilirubin, a yellow pigment, from the blood. In addition, bilirubin is produced in larger than normal amounts in the immediate days after birth. Severe neonatal (newborn) jaundice can produce significant and irreversible central nervous system damage. Bilirubin results from the breakdown of heme, a component of the oxygen-carrying molecule hemoglobin, in red blood cells. Studies on heme degradation to form bilirubin led Attallah Kappas (1926 - ) and colleagues to undertake the development of synthetic heme analogues that could strongly inhibit the enzyme, heme oxygenase, that controls this process. They reasoned that blocking the enzyme would slow the conversion of heme to bilirubin in newborns and prevent bilirubin concentrations from reaching toxic levels. Heme oxygenase was first isolated and purified from liver by the Kappas group in 1974. Its recognition as the enzyme that controls the rate of heme degradation to bilirubin clarified the mechanism of this process, which had been incorrectly attributed by earlier investigators to a different enzyme—one related to the cytochrome P450 system.
Current management of severe neonatal jaundice involves exposing infants to prolonged, intensive light therapy. This treatment is administered to several hundred thousand of the more than 4,000,000 babies born annually in the United States. Phototherapy is successful in reducing blood bilirubin levels in jaundiced babies but it is a slow, cumbersome, and time-consuming procedure and requires close observation of infants under treatment. In large parts of the underdeveloped world, this methodology is not available, among other reasons because of the lack of available electricity. A decisive advance in clinical management of this problem was achieved when an effective, safe heme oxygenase inhibitor, Sn-mesoporphyrin (SnMP), was developed by Kappas and his colleagues. SnMP acts as a potent, rapidly acting, competitive substrate for heme oxygenase, blocking the binding of natural heme to the enzyme thus inhibiting bilirubin formation. The fraction of natural heme not converted to bilirubin is excreted intact. SnMP is administered as a single, small dose of medication and its effect lasts through the period after birth during which the newborn is at special risk for developing significant jaundice. The inhibitor can be given preventively at birth or can be administered later, since it promptly interdicts the progression of hyperbilirubinemia at any time in the course of its development that the clinical situation warrants.
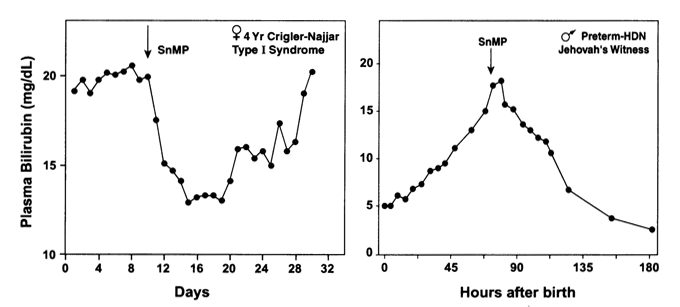
Effect of a single dose of SnMP on plasma bilirubin levels in a pre-term newborn with progressive hyperbilirubinemia due to a hemolytic process (right panel). From Pediatrics, 2004, 113: 119-123
SnMP has undergone extensive clinical trials in jaundiced babies in the United States and in a number of foreign countries extending from Argentina to Greece to Viet Nam. It is currently undergoing pharmaceutical development in the United States and in Europe. The success of this research effort represents a complete example of the translational process in the basic and clinical sciences, extending from clinical observation, to laboratory investigation, to clinical application.
Attallah Kappas received an AB degree from Columbia (1947), following completion of his military service; and an MD degree from the University of Chicago (1950). He undertook post-graduate training at the Memorial Sloan-Kettering Cancer Center (MSKCC) and the Peter Bent Brigham Hospital, returning to Chicago in 1957. He was recruited to The Rockefeller University in 1967. In addition to heading the Laboratory of Pharmacology, he served successively during the period 1971-1991 as Program Director; Physician-in-Chief; and Vice President. He was a strong advocate of developing close collaborative relationships with Rockefeller's neighboring institutions and held for various periods joint appointments as the Vincent Astor Chair in Clinical Sciences at MSKCC; professorships in Medicine and Pharmacology at Cornell University Medical College; and Co-Directorship of the Rockefeller-Cornell Medical College MD-PhD program. He was a recipient of Commonwealth Fund and John Simon Guggenheim Memorial Foundation Fellowships; The Burroughs Wellcome Fund Special Award in Clinical Pharmacology; and the ASPET Award for Research in Therapeutics of the American Society of Pharmacology and Experimental Therapeutics. He served as an editor of the Journal of Experimental Medicine during 1971-1981. He was appointed the first Nicholson Exchange Professor from Rockefeller to the Karolinska Institute in 1985 and in 1989 became the first recipient of the newly established NIH Award for Excellence in Clinical Research. He is presently Sherman Fairchild Professor and Physician-in-Chief, Emeritus, at The Rockefeller University.
Selected Publications
Maines MD and Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci USA, 1974, 71: 4293-4297
http://www.pnas.org/content/71/11/4293.full.pdf+html
Drummond GS and Kappas A. Prevention of neonatal hyperbilirubinemia by tin-protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci USA, 1981, 78: 6466-6470
http://www.pnas.org/content/78/10/6466.full.pdf+html
Drummond GS, Galbraith RA, Sardana MK, and Kappas A. Reduction of the C2 and C4 vinyl groups of Sn-protoporphyrin to form Sn-mesoporphyrin markedly enhances the ability of the metalloporphyrins to inhibit in vivo heme catabolism. Arch Biochem Biophysics, 1987, 255: 64-74
Kappas A, Drummond GS, Henschke CI, and Valaes T. Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics, 1995, 95: 468-474
Valaes, T, Drummond GS, and Kappas A. Control of hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns using an inhibitor of bilirubin production, Sn-mesoporphyrin. Pediatrics, 1998, 101, No. 5 e1-7
http://pediatrics.aappublications.org/cgi/reprint/101/5/e1
Kappas A, Drummond GS, and Valaes T. A single dose of Sn-mesoporphyrin prevents development of significant hyperbilirubinemia in glucose-6 phosphate dehydrogenase deficient newborns. Pediatrics, 2001, 108: 25-30
http://pediatrics.aappublications.org/cgi/reprint/108/1/25
Kappas A. A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics, 2004, 113: 119-123
http://pediatrics.aappublications.org/cgi/reprint/113/1/119
Principal members of the Kappas laboratory group who participated in these research programs were: Nader G. Abraham, Alvito P. Alvares, Karl E. Anderson, David R. Bickers, Allan H. Conney, George S. Drummond, Richard A. Galbraith, Mahin D. Maines, Arlene B. Rifkind, Daniel W. Rosenberg, Mohinder K. Sardana, Shigeru Sassa and Takeo Yoshinaga.
Links
Attallah Kappas Laboratory
http://www.rockefeller.edu/labheads/kappas/kappas-lab.php
Learn More About the Laboratory of Pharmacology
At Rockefeller Attallah Kappas established the Laboratory of Pharmacology in the Hospital in 1967. His group focused on research in heme biology, and undertook a broadly based program of biochemical and clinical studies of each of the enzymes involved in heme synthesis and the porphyrias related to them; on heme utilization in the form of cytochrome P450 in liver and the regulation of P450-mediated drug and hormone metabolism; and on the development and clinical application of synthetic heme analogues for regulating the catabolism of heme to the bile pigment bilirubin. In each main direction of his biochemical research Dr. Kappas conducted a major program of clinical investigation. He also established at the Rockefeller Hospital a center for the diagnosis, care, and study of the diseases of porphyrin origin and, in collaborative relationships with other institutions, undertook similar efforts for studies of newborns and children with genetic or developmental forms of severe jaundice. Principal members of his laboratory group who joined in these research programs were: Nader G. Abraham, Alvito P. Alvares, Karl E. Anderson, David R. Bickers, Allan H. Conney, George S. Drummond, Richard A. Galbraith, Mahin D. Maines, Daniel W. Rosenberg, Mohinder K. Sardana, and Shigeru Sassa.