Pioneering the Genome-Wide Association Study and Identifying a Cause of Age-Related Macular Degeneration

Ott, Jurg
Finding the many genetic mutations that contribute to common, complex diseases like diabetes and heart disease once seemed like a needle-in-a-haystack proposition. But Rockefeller's Jurg Ott (1939 - ) has led researchers in developing a new technique called a genome-wide association study (GWAS) for finding genetic differences between people with such complex diseases and in healthy individuals. In a landmark proof-of-concept study in 2005 he and colleagues identified a genetic variation strongly associated with age-related macular degeneration (AMD), the most common cause of irreversible vision loss in the developed world, which affects some 10 million Americans. This research opened the door to hundreds of subsequent studies by scientists who have since used GWAS to find genomic variations that underlie disease, and that also explain differences in how people respond to drug therapies.
This method scans the three billion nucleotides in an individual's genome sequence for the single-nucleotide polymorphisms (SNPs), or substitutions, that occur about once in every 1,000 nucleotides. Most of these small changes have no effect on health. So scientists must analyze hundreds of thousands of variations among a large group of people to find changes that occur in people with a disease but not in healthy people. Ott's laboratory has led the field in developing statistical and computational techniques for such analysis. In addition, scientists once thought it would be necessary to scan the genomes of thousands of people in order to find associations—a daunting prospect that would severely limit the utility of the technique. But by choosing well-matched cases and controls Ott, former research assistant professor Josephine Hoh, and colleagues showed that a case-control GWAS could be performed successfully with only 96 people with severe cases of AMD and 50 people matched for age, gender, and many environmental factors. They discovered that the so-called dry form of AMD is associated with a genetic variation on chromosome 1 that changes the sequence of an immune system protein involved in inflammation called human complement factor H. Prior to this study, no one had suggested that a complement factor was associated with the disease. Hoh is now an associate professor at Yale University.
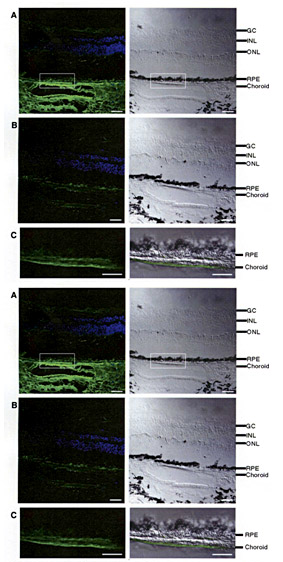
Immunofluorescence localization of CFH protein in human retina. From Science, 2005, 308: 385-389
Before turning to case-control association analysis, Ott was a founder of the field of genetic linkage, another approach to locating genes on chromosomes and ultimately identifying those that contribute to disease. He authored the first publicly accessible computer program on human linkage analysis (dubbed LIPED). His work led to some of the first methods for computer simulation in family pedigrees and provided the statistical framework underlying the newer approaches to haplotype relative risk methods that have become important tools in the search for disease-marker associations. He has analyzed gene linkages for a number of other disorders, including hypertension, Creutzfeldt-Jacob disease, multiple sclerosis, and retinitis pigmentosa.
Ott's laboratory continues to focus on the interpretation of genomic data by developing new mathematical-statistical methods for human gene mapping and building computer programs to implement them. He uses the resulting information to study the interactions among multiple disease loci that underlie complex traits, as well as to study how environmental risk factors modify disease loci effects. Ott collaborates with other researchers on the analysis of their genetic data and maintains a web/ftp site for disseminating information and computer programs.
Ott received the PhD from the University of Zürich (1967), taught physics at a state college in Switzerland, and then worked as a biostatistician until 1970. He earned his master's degree in biomathematics from the University of Washington, Seattle (1972), then stayed on at the university, advancing to associate professor. Ott returned to Zürich in 1979 as the assistant director for the city's statistics office. In 1986 he accepted two positions in New York City: professor of genetics and development at Columbia University and research scientist and director of the department of statistics at the New York State Psychiatric Institute. Ott joined Rockefeller in 1996 as professor and head of the Laboratory of Statistical Genetics. He maintains an office at the Beijing Institute of Genomics, Chinese Academy of Sciences. His achievements have been recognized with the Medal of Honor from the German Society for Human Genetics (2007), and the Ming Tsuang Lifetime Achievement Award from the International Society of Psychiatric Genetics (2008). He received a MERIT award from the National Institutes of Mental Health (2001) and is a member of the American Association for the Advancement of Science and the Human Genome Organization.
Selected Publications
Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, and Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science, 2005, 308:385-389
Hoh J and Ott J. Mathematical multi-locus approaches to localizing complex human trait genes. Nat Rev Genet, 2003, 4: 701-709
Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, and Hoh J. Htra1 promoter polymorphism in wet age-related macular degeneration. Science, 2006, 314: 989-992
Further Reading
Ott J. Analysis of Human Genetic Linkage, third edition. Baltimore: Johns Hopkins Univ Press, 1999
Terwilliger JD and Ott J. Handbook of Human Genetic Linkage. Baltimore: Johns Hopkins Univ Press, 1994
Links
Jurg Ott, Laboratory of Statistical Genetics
http://linkage.rockefeller.edu/ott/
Josephine Hoh, Yale University
http://variation.yale.edu/