Opening the Field of Intracellular Protein Traffic

Blobel, Gunter
An average human cell contains a billion protein molecules, and these are constantly being manufactured, sorted, and distributed to the structures within the cell where they do their work. Günter Blobel (1936- ) joined the Rockefeller laboratory of George Palade (1912-2008) in 1967. Palade, who received a Nobel Prize in 1974, discovered ribosomes, the cellular elements that manufacture proteins. In the 1960s Palade's laboratory worked out the intracellular pathway by which proteins are secreted from cells, showing that the first step in this pathway is translocation of secretory proteins across the endoplasmic reticulum (ER) followed by their vesicular transport and exocytosis at the plasma membrane. The underlying biochemical mechanisms remained unknown. Blobel's research in the following decades revealed these mechanisms. In 1999 he was awarded the Nobel Prize for "the discovery that proteins have intrinsic signals that govern their transport and localization in the cell."
In 1971 Blobel and David Sabatini first proposed the idea that every secretory protein contains a short sequence of amino acids at its N-terminal end serving as a zip code to direct it to the ER membrane. In 1975, Blobel proposed the signal hypothesis in which the short N-terminal sequence of the nascent secretory polypeptide chain, now dubbed signal sequence, would be recognized by cellular recognition factors and would be targeted to the ER, where it would open a protein-conducting channel in the ER. He also proposed that nascent membrane proteins would contain a signal sequence to initiate translocation and a subsequent "stop transfer sequence" that would open the protein conducting channel laterally, toward the lipid bilayer, yielding integration of the nascent transmembrane protein into the lipid bilayer. Hence the asymmetric integration of membrane proteins into the lipid bilayer is not a "spontaneous" event but is catalyzed and dictated by sequence elements of the membrane protein that are decoded by the protein conducting channel.
Moreover, Blobel extended these ideas to proteins that have to cross or to be integrated into membranes of other cellular organelles. By setting up in vitro systems, Blobel and coworkers were able to provide experimental evidence for these proposals. They identified key elements, including the signal recognition particle (SRP), the SRP receptor in the ER, a new class of enzymes called signal peptidases, and a signal sequence-gated protein conducting channel.
The techniques Blobel developed to study translocation are now used in laboratories around the world. He and others have demonstrated that proteins destined for different organelles—the mitochondria, chloroplasts, and so on—are tagged with specialized topogenic signals. These signals also have been shown to be universal in living cells. Beyond resolving a basic biological problem, Blobel's research has furthered scientific understanding of human physiology and pathology. Mutations in topogenic signals—which lead to mistargeted proteins—underlie several inherited diseases. Recent research in Blobel's laboratory has focused on how molecules traverse the two membranes of a cell's nucleus through nuclear pore complexes.
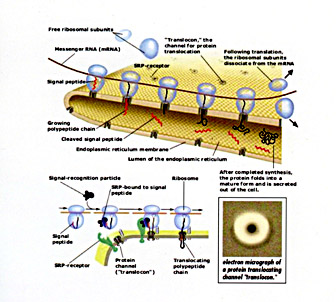
Protein translocation across recticulum membrane. Courtesy of the Nobel Committee for Physiology or Medicine, 1999
Günter Blobel received the MD from the University of Tubingen (1960) and the PhD from the University of Wisconsin, Madison (1967), where he worked with Van R. Potter. Beginning with a postdoctoral fellowship in 1967 in the laboratory of George Palade, he has remained at The Rockefeller University his entire career. Blobel was appointed professor in 1976, John D. Rockefeller, Jr., professor in 1992, and an investigator of the Howard Hughes Medical Institute in 1986. In addition to the Nobel Prize in Physiology or Medicine (1999), his work has been recognized with the Gairdner Foundation International Award (1982), the Louisa Gross Horwitz Prize (1987), the King Faisal Award (1996), and the Lasker Award (1993). He is a member of the U.S. National Academy of Sciences (1983), the American Philosophical Society, the Pontifical Academy of Sciences and the German Orden pour le Mérite.
Selected Publications
Blobel G and Sabatini DD. Ribosome-membrane interaction in eukaryotic cells. Biomembranes, 1971, 2: 193-195
Blobel G and Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol, 1975, 67: 835-851
http://jcb.rupress.org/cgi/reprint/67/3/835
Blobel G and Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol, 1975, 67: 852-862
http://jcb.rupress.org/cgi/reprint/67/3/852
Dobberstein B, Blobel G, and Chua NH. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA, 1977, 74:1082-1085
http://www.pnas.org/content/74/3/1082.full.pdf+html
Katz FN, Rothman JE, Lingappa VR, Blobel G, and Lodish HF. Membrane assembly in vitro: Synthesis, glycosylatio, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci USA, 1977, 74:3278-3282
http://www.pnas.org/content/74/8/3278.full.pdf+html
Maccecchini ML, Rudin Y, Blobel G, and Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci USA, 1979, 76: 343-347
http://www.pnas.org/content/76/1/343.full.pdf+html
Walter P and Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature, 1982, 299: 691-698
Gilmore R, Walter P, and Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol, 1982, 95: 470-477
http://jcb.rupress.org/cgi/reprint/95/2/470
Evans EA, Gilmore R, and Blobel G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci USA, 1986, 83: 581-585
http://www.pnas.org/content/83/3/581.full.pdf+html
Davis LI and Blobel G. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc Natl Acad Sci USA, 1987, 84: 7552-7556
http://www.pnas.org/content/84/21/7552.full.pdf+html
Simon SM and Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell, 1991, 65: 371-380
Moore MS and Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature, 1993, 365: 661-663
Melcák I, Hoelz A, and Blobel G. Structure of Nup58/45 suggests flexible nuclear pore diameter by intermolecular sliding. Science, 2007, 315: 1729-1732
Hsia KC, Stavropoulos P, Blobel G, and Hoelz A. Architecture of a coat for the nuclear pore membrane. Cell, 2007, 131: 1313-1326
Further Reading
Blobel G. Protein targeting (Nobel lecture). Chembiochem, 2000, 1: 86-102
Links
The Rockefeller University Laboratory of Cell Biology
http://www.rockefeller.edu/labheads/blobel/blobel-lab.php
Animation: Protein Targeting and Translocation
http://www.rockefeller.edu/pubinfo/proteintarget.html
The Nobel Prize in Physiology or Medicine, 1999
http://nobelprize.org/nobel_prizes/medicine/laureates/1999/index.html
Interview with Günter Blobel
http://nobelprize.org/nobel_prizes/medicine/laureates/1999/blobel-interview.html
Albert Lasker Basic Medical Research Award, 1993
http://www.laskerfoundation.org/awards/1993basic.htm