From a Rare Blood Platelet Disorder to a New Treatment for Heart Attack
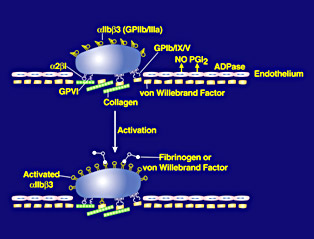
Human blood platelets, small cell fragments, are required to prevent bleeding. The platelets stick to sites of blood vessel injury almost immediately and then bind to each other, or aggregate, to create the initial framework for a blood clot. Unfortunately, this same process can close off a blood vessel and cause a heart attack or stroke if an artery is narrowed by lipid deposition (atherosclerosis) and the atherosclerotic material ruptures and platelets deposit and form aggregates.

Coller, Barry
In the early 1980s Barry Coller (1945 - ) and his colleagues were among the first to apply the then new technology of monoclonal antibodies to the study of blood platelets. They began by injecting human platelets into a mouse. The mouse reacted as it would to an invading germ, by making antibody molecules that bound to different parts of the platelets, thus targeting the invader for rapid removal from the body. Coller discovered that one of the antibodies could completely block platelet aggregation by binding to a receptor termed GPIIb/IIIa or αIIbβ3, which acts as a docking station for molecules that bridge between platelets to form the aggregates. When the antibody bound to the receptor, the bridging molecule, fibrinogen, could not. These antibodies provided valuable new information about the structure and function of the receptors and were used to help diagnose Glanzmann thrombasthenia, a very rare bleeding disorder in which patients either lack or have abnormal αIIbβ3 receptors. The antibodies were also used for prenatal diagnosis of the disorder for families that wanted the information.
The antibodies inhibited platelet function to a greater extent than aspirin, which is often recommended to prevent blood clot formation inside arteries. So Coller's group also investigated whether modified versions of the antibodies could protect animals from developing heart attacks and strokes in model systems. These studies were encouraging, and in collaboration with scientists at a new biotechnology company, Centocor, Coller developed a derivative of one of the antibodies for human use. The resulting drug, abciximab, was shown to protect patients with heart disease from dying or having a heart attack when they underwent angioplasty and stent placement to open up their clogged coronary arteries. From the time of its approval by the U.S. Food and Drug Administration in 1994 until the end of 2008, more than 2.5 million people had been treated with abciximab.
Platelet activation and ligand binding
Barry S. Coller received his BA from Columbia University (1966) and his MD from New York University School of Medicine (1970), and trained in internal medicine at Bellevue Hospital in New York and hematology at the National Institutes of Health in Bethesda. From 1976 to 1993 his academic and administrative positions at Stony Brook University included Professor of Medicine and Pathology and Chief of Hematology. From 1993 to 2001 he served as Chair of Medicine at Mount Sinai School of Medicine. Coller came to Rockefeller in 2001 as the first David Rockefeller Professor of Medicine, head of the Laboratory of Blood and Vascular Biology, physician-in-chief of The Rockefeller University Hospital and vice president for medical affairs. His research interests include platelet physiology, hemostasis, and thrombosis; his translational research projects include the development of new antiplatelet agents to prevent and treat thrombosis, and a new diagnostic device to monitor antiplatelet therapy. Coller's work has been recognized by a National Research Achievement Award from the American Heart Association (1998), the Warren Alpert Foundation Award (2001), and the Robert J. and Claire Pasarow Foundation Award (2005). He is a member of the Institute of Medicine, the U.S. National Academy of Sciences, and the American Academy of Arts and Sciences.
Selected Publications
Coller BS. Platelet GPIIb/IIIa antagonists: The first anti-integrin receptor therapeutics. J Clin Invest, 1997, 99:1467-1471
Coller BS. From Bench to bedside. Blockade of platelet GPIIb/IIIa receptors as an antithrombotic strategy. Circulation, 1995, 92: 2373-2380
Newman PJ, Seligsohn U, Lyman S, and Coller BS. The molecular genetic basis of Glanzmann thrombasthenia in the IraqiJewish and Arab populations in Israel. Proc Natl Acad Sci USA, 1991, 88: 3160-3164
Gold HK, Gimple LW, Yasuda T, Leinbach R, Werner W, Holt R, Jordan R, Berger H, Collen D, and Coller BS. Pharmacodynamic study of F(ab')2 fragments of murine monoclonal antibody 7E3 directed against human platelet glycoprotein IIb/IIIa, in patients with unstable angina pectoris. J Clin Invest, 1990, 86: 651-659
Coller BS. A new murine monoclonal antibody reports an activation dependent change in the conformation and/or microenvironment of the platelet GPIIb/IIIa complex. J Clin Invest, 1985, 76: 101-108
Coller BS and Scudder LE. Inhibition of dog platelet function by in vivo infusion of F(ab')2 fragments of a monoclonal antibody. Blood, 1985, 66: 1456-1459
Seligsohn U, Mibashan RS, Rodeck CH, Nicolaides KH, Millar DS, and Coller BS. Prenatal diagnosis of Glanzmann's thrombasthenia. Lancet, 1985, 2: 1419
Further Reading
Coller BS and Shattil SJ. The GPIIb/IIIa (integrin αIIbβ3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood, 2008, 112: 3011-3025
Links
Laboratory of Blood and Vascular Biology
http://www.rockefeller.edu/labheads/coller/intro.php