Form and Function: The First Sequence of an Enzyme, Ribonuclease

Moore, Stanford and Stein, William
Courtesy of The Rockefeller Archive Center
Understanding the relationships between protein structure and function underlies a broad swath of research on the basic biology of health and disease, as well as the rational design of new drugs. When Stanford Moore (1913-1982) and William Stein (1911-1980) began their lifelong scientific collaboration in 1939, chemists were only beginning to conceive of proteins as chains of amino acids linked together in unique sequences. Over the next 20 years Moore and Stein developed methods for analyzing amino acids, and in 1960 they published the first sequence of an enzyme: the 124-amino-acid long protein termed ribonuclease (RNase) for its ability to cleave molecules of RNA. But an amino acid sequence alone tells little about how such a protein works-unlike letters of the alphabet that are able to create words in 2-dimensional space, the amino acids in proteins fold into complex 3-dimensional structures with hills, valleys, and crevices of varying electrical charge and properties. Moore and Stein went on to describe the chemistry of ribonuclease's business end-its "active site" where the cleavage of RNA occurs-even before the molecule's shape was known. For their contribution to the understanding of the chemical structure and catalytic activity of the active center of the ribonuclease molecule, they were awarded the Nobel Prize in 1972, shared with Christian B. Anfinsen.
Before Moore and Stein could tackle the problem of ribonuclease structure, however, they had to invent new analytical techniques. Their contributions to this field, during the heyday of chemical separation methods, made it possible for investigators everywhere to quantitatively study the amino acids in digested protein, body fluids, and other biological materials. Moore and Stein began by improving existing chromatographic methods for separating amino acids with starch columns, and invented a photoelectric drop-counting fraction collector, a vital piece of equipment that remains in widespread use today in labs around the world. Later they used ion-exchange chromatography, and with Daryl Spackman they invented an automated amino acid analyzer. Moore and Stein never sought patents for their instruments, which were widely adopted by other scientists and served as prototypes for commercial models.
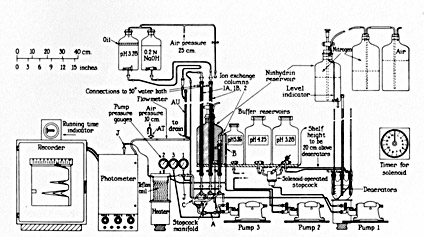
Schematic diagram of the initial design of an automatic amino acid analyzer (1958)
Other scientists had studied ribonuclease before Moore and Stein, including scientists at Rockefeller. Rene Dubos and R.H.S. Thompson had partially purified it in 1938, and two years later Moses Kunitz crystallized the enzyme. Later, at the same time as Moore and Stein determined ribonuclease's amino acid sequence, Bruce Merrifield (1921-2006) at Rockefeller was developing his method for solid phase peptide synthesis. By 1969 he was able to use this exciting new technique to link amino acids together in the proper sequence to artificially synthesize ribonuclease. In 1984, Merrifield also received the Nobel Prize.
Stanford Moore received the BA from Vanderbilt University (1935) and the PhD from the University of Wisconsin (1938). He joined Max Bergmann's Rockefeller laboratory in 1939. From 1942 to 1945 he served on the National Defense Research Committee, Office of Scientific Research and Development, and in 1950 he did research in Cambridge, England, with Frederick Sanger. Moore and Stein were made heads of their own joint laboratory in 1945, and aside from these interruptions, Moore remained at Rockefeller his entire career. In addition to the Nobel Prize in Chemistry (1972) Moore and Stein shared many awards, including the American Chemical Society Award in Chromatography and Electrophoresis (1964), the Richards Medal of the American Chemical Society (1972), and the Kaj Linderstrøm-Lang Medal, Copenhagen (1972). Moore served as president of the American Society of Biological Chemists (1966-1967) and was an elected member of the U.S. National Academy of Sciences (1960) and the American Academy of Arts and Sciences (1960).

Moore, Stanford and Stein, William
Courtesy of The Rockefeller Archive Center
William H. Stein received his undergraduate degree from Harvard University (1933) and the PhD from Columbia University (1937). In 1938 Stein joined the Rockefeller laboratory of chemist Max Bergmann. He and Moore were made joint heads of their own laboratory in 1945, after Bergmann's death in 1944. Stein remained at Rockefeller his entire career. He concluded many years of service to the Journal of Biological Chemistry as its editor from 1968 to 1971. Stein and Moore shared many awards, including the American Chemical Society Award in Chromatography and Electrophoresis (1964), the Richards Medal of the American Chemical Society (1972), and the Kaj Linderstrøm-Lang Medal, Copenhagen (1972). Stein was an elected member of the U.S. National Academy of Sciences and the American Academy of Arts and Sciences.
Selected Publications
Stein WH and Moore S. Chromatography of amino acids on starch columns. Separation of phenylalanine, leucine, isoleucine, methionine, tyrosine, and valine. J Biol Chem, 1948, 176: 337-365
http://www.jbc.org/cgi/reprint/176/1/337
Moore S and Stein WH. Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem, 1948, 176: 367-388
http://www.jbc.org/cgi/reprint/176/1/367
Moore S, Spackman DH, and Stein WH. Chromatography of amino acids on sulfonated polystyrene resins. An improved system. Anal Chem, 1958, 30: 1185-1190
Spackman DH, Stein WH, and Moore S. Automatic recording apparatus for use in the chromatography of amino acids. Anal Chem, 1958, 30:1190-1206
Hirs CHW, Moore S, and Stein WH. The sequence of the amino acid residues in performic acid-oxidized ribonuclease. J Biol Chem, 1960, 235(3): 633-647
http://www.jbc.org/cgi/reprint/235/3/633
Spackman DH, Stein WH, and Moore S, with the assistance of Zamoyska AM. The disulfide bonds of ribonuclease. J Biol Chem, 1960, 235(3): 648-659
http://www.jbc.org/cgi/reprint/235/3/648
Smyth DG, Stein WH, and Moore S. The sequence of amino acid residues in bovine pancreatic ribonuclease: revisions and confirmations. J Biol Chem, 1963, 238: 227-234
http://www.jbc.org/cgi/reprint/238/1/227
Crestfield AM, Stein WH, and Moore S. Alkylation and identification of the histidine residues at the active site of ribonuclease. J Biol Chem, 1963, 238: 2413-2420
http://www.jbc.org/cgi/reprint/238/7/2413
Moore S and Stein WH. Chemical structures of pancreatic ribonuclease and deoxyribonuclease. Science, 1973, 180: 458-464
Further Reading
Dubos RJ and Thompson RHS. The decomposition of yeast nucleic acid by a heat-resistant enzyme. J Biol Chem, 1938, 124: 501-510
http://www.jbc.org/cgi/reprint/124/2/501
Kunitz M. Crystalline ribonuclease. J Gen Physiol, 1940, 24: 15 -32
http://jgp.rupress.org/cgi/reprint/24/1/15
Moore S. William H. Stein (1911-1980): A Biographical Memoir. Washington, DC: U.S. National Academy of Sciences, 1987, 56: 414-441
Smith E and Hirs CHW. Stanford Moore (1913-1982): A Biographical Memoir. Washington, DC: U.S. National Academy of Sciences, 1987, 56: 354 -385
Moore S. William H. Stein. J Biol Chem, 1980, 255: 9517-9518
http://www.jbc.org/cgi/reprint/255/20/9517
Kresge N, Simoni RD, and Hill RL. The fruits of collaboration: Chromatography, amino acid analyzers, and the chemical structure of ribonuclease by William H. Stein and Stanford Moore. J Biol Chem, 2005, 280: e6
http://www.jbc.org/cgi/content/full/280/9/e6
Kresge N, Simoni RD, and Hill RL. The elucidation of the structure of ribonuclease by Stanford Moore and William H. Stein. J Biol Chem, 2005, 280: e47
http://www.jbc.org/cgi/content/full/280/50/e47
Links
The Nobel Prize in Chemistry, 1972
http://nobelprize.org/nobel_prizes/chemistry/laureates/1972/