Developing Effective Vaccines Against Group A and Group C Meningitis

Gotschlich, Emil
Since the Rockefeller Institute's inception, researchers there endeavored to treat and prevent meningitis caused by meningococcal bacteria. Early in the twentieth century Simon Flexner developed horse antisera that saved the lives of many patients. In the 1920s the seminal work of Oswald Avery, Michael Heidelberger, and Walter Goebel demonstrated that the virulence of pneumococci, which caused pneumonia, was attributable to the presence of capsules on the surface of the bacteria and that these were complex polysaccharides. This finding was extended to other bacteria, and in 1935 Henry Scherp and Geoffrey Rake, working in the laboratory of Leslie T. Webster, isolated the capsular substance of the predominant meningococcus serotype A (then called type I). They demonstrated that it was a polysaccharide composed of a nitrogen-containing sugar and phosphoric acid units. In the 1930s and 1940s, other Rockefeller researchers showed that polysaccharides were potent antigens, and used them in early efforts to develop a pneumonia vaccine. Then, in the late 1960s, Emil C. Gotschlich (1935 - ), a member of the laboratory of Maclyn McCarty, built on these decades of Rockefeller research to achieve the goal of developing effective vaccines against meningitis.
Meningococcal meningitis was a chronic problem at military recruit camps. From 1966 to 1968 Gotschlich left the University for military duty at the Walter Reed Army Institute of Research, where he developed novel methods to isolate highly purified meningococcal capsular polysaccharides from serotypes A, B, and C that preserved their high molecular weight. With a team of colleagues he showed that injection of 50 µg of group A or group C polysaccharide induced human beings to rapidly produce specific antibodies, and that these were able to kill meningococci in vitro. The Army research team in 1970 demonstrated that the group C polysaccharide was 90 percent effective in preventing meningitis by this organism in military recruits.
The efficacy of the group A polysaccharide could not be tested in the U.S. since disease due to that serotype was very rare. However, group A meningitis was very common in other parts of the world. In a collaboration with the World Health Organization and the Institut Merieux, a French vaccine manufacturer, lots of group A vaccine were prepared. The vaccine's efficacy in preventing meningitis was demonstrated in controlled field trials first in Egypt and the Sudan and then in Finland.
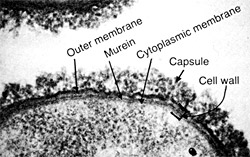
Electron-micrograph (taken by Dr. John Swanson) of a Group A meningococcus shows the capsule, enclosing the entire wall, and the cell wall.
The group A and group C vaccines were approved by the U.S. Food and Drug Administration in 1977. They were the first vaccines to be standardized solely on the basis of physical and chemical criteria rather than on their biological activity in animals, a practice that has become increasingly the norm for modern vaccines. Billions of people have received the group A and group C vaccines, particularly in China, Egypt, Saudi Arabia (which requires all pilgrims entering the country on Hajj to be vaccinated), and in Africa when epidemic disease outbreaks occur. Recently the meningococcal polysaccharides have been linked to proteins to prepare conjugate vaccines. This markedly improves their immunogenicity in infants and now allows disease prevention at all ages.
Emil C. Gotschlich received the MD from the New York University School of Medicine (1959). He interned at Bellevue Hospital in New York before joining The Rockefeller University's Laboratory of Bacteriology and Immunology in 1960 under the co-leadership of Maclyn McCarty and Rebecca C. Lancefield. He was promoted to professor and senior physician at The Rockefeller University Hospital in 1978. From 1996 to 2005, he served as the hospital's vice president for medical sciences. Gotschlich's achievements have been recognized by numerous awards and honors, including the Albert Lasker Award for Clinical Research (1978). He is a member of the U.S. National Academy of Sciences and its Institute of Medicine.
Selected Publications
Gotschlich EC, Liu TY, and Artenstein MS. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med, 1969, 129:1349-1366
http://jem.rupress.org/cgi/reprint/129/6/1349
Gotschlich EC, Goldschneider I, and Artenstein MS. Human immunity to the meningococcus. 4. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med, 1969, 129:1367-1384
http://jem.rupress.org/cgi/reprint/129/6/1367
Gotschlich EC, Goldschneider I, and Artenstein MS. Human immunity to the meningococcus. 5. The effect of immunization with meningococcal group C polysaccharide on the carrier state. J Exp Med, 1969, 129:1385-1395
http://jem.rupress.org/cgi/reprint/129/6/1385
Gotschlich EC, Rey M, Triau R, and Sparks KJ. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest, 1972, 51: 89-96
http://www.jci.org/articles/view/106801/pdf
Wahdan MH, Rizk F, el-Akkad AM, el-Ghoroury AA, Hablas R, Girgis NI, Amer A, Boctar W, Sippel JE, Gotschlich EC, Triau R, Sanborn WR, and Cvjetanovic B. A controlled field trial of a serogroup A meningococcal polysaccharide vaccine. Bull World Health Organ, 1973, 48: 667-673
http://whqlibdoc.who.int/bulletin/1973/Vol48/Vol48-No6/
Lepow ML, Goldschneider I, Gold R, Randolph M, and Gotschlich EC. Persistence of antibody following immunization of children with groups A and C meningococcal polysaccharide vaccines. Pediatrics, 1977, 60: 673-680.
http://pediatrics.aappublications.org/content/vol60/issue5/index.dtl
Further Reading
Pollard AJ, Perrett KP, and Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol, 2009, 9: 213-220
Links
Emil C. Gotschlich, Laboratory of Bacterial Pathogenesis
http://www.rockefeller.edu/labheads/gotschlich/gotsch_fisch.php
1978 Lasker Awards
http://www.laskerfoundation.org/awards/1978clinical.htm